The longstanding mystery of how soot forms—which combustion scientists have been trying to solve for decades—appears to be finally solved, thanks to a research team led by Sandia National Laboratories.
Soot is ubiquitous and has harmful effects on human health, agriculture, energy-consumption efficiency, the climate, and air quality. It is said to be responsible for a significant increase in the rates of cardiovascular and pulmonary diseases and associated deaths, as well as millions of deaths worldwide, largely from indoor cooking and heating in developing nations.
“Understanding soot formation gives us a better chance of reducing its emissions from engines, forest fires, and cook stoves, and control its production and characteristics during industrial processes,” says Sandia researcher Hope Michelsen. Everyone knows what soot is, but no one could explain how gaseous fuel molecules become solid soot particles.”
Soot formation turns out to be different from the typical process in which gas molecules condense into a particle. Instead, it requires fast chemical reactions rather than condensation. The same physics could also apply to other high-temperature conditions, such as interstellar space, where large quantities of carbon-dust particles are formed.
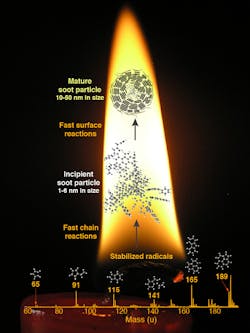
The mass spectrum at the bottom of the flame shows peaks for radicals driving the soot-forming reaction. The incipient soot particle shown between the two arrows is the cluster that marks a transition to the condensed phase. Fast reactions grow the particle shown at the top of the flame.
Soot forms when hydrocarbon fuels such as oil, natural gas, and wood are burned. Although it has harmful health and environmental effects, soot is extremely important to many industrial processes, such as boiler performance, glass production, and making carbon-black for reinforcing rubber products such as tires and for pigments.
In its final form, soot is a solid-like graphite, but it is initially formed from gaseous hydrocarbons. Experimental evidence indicates that it goes from a gas to a liquid before it becomes a solid. Scientists have been trying for decades to explain that transition.
Soot particles form when gaseous molecules are heated to high temperatures, and they don’t easily turn back into gaseous molecules the way water droplets do when they are heated. Strong chemical bonds hold soot particles together.
Scientists have long suspected that chemical bonds must be formed to make soot. However, soot formation is fast, and researchers did not understand how the required chemical bonds could form so quickly. To make the problem even more difficult, researchers weren’t even sure which gas-phase molecules were involved in producing soot.
Sandia National Laboratories researchers (l to r) Paul Schrader, Hope Michelsen, and Olof Johansson cracked the code to soot formation. (Photo by Brent Haglund)
The key to soot formation, it turns out, is resonance-stabilized radicals. In general, molecules that are radicals have unpaired electrons that they want to share, which makes them reactive. But, unlike most radicals, these resonance-stabilized radicals have unpaired electrons that participate in other bonds in the molecule. Sharing electron density between unpaired electrons and other bonds in the molecule makes these radicals more stable than others, but nevertheless, they are more reactive than most of the other large molecules that form soot. Measurements conducted at the Advanced Light Source, a DoE facility at Lawrence Berkeley Lab, showed a sequence of these radical species in all the flames studied.
“We determined that these radicals can start a chain reaction,” says Michelsen.
When these radicals react with other molecules, they easily form new resonance-stabilized radicals. In the process, they react with other gaseous hydrocarbons and keep growing, regenerating radicals as part of the growing particle.