Storing Hydrogen for Fuel-Cell Vehicles in Solid-State Compounds
Scientists at the Lawrence Livermore National Laboratory are exploring ways to use an inexpensive and layered superconductor compound to efficiently store hydrogen. They have already discovered the key mechanism used by magnesium diboride (MgB2) to absorb hydrogen and the reaction pathway that converts MgB2 to its highest hydrogen capacity form, magnesium borohydride (Mg(BH4)2). Mg(BH4)2 is a particularly promising hydrogen storage material because of its high hydrogen content and attractive thermodynamics.
Storing hydrogen is critical for hydrogen-fueled transportation as well as grid resiliency, energy storage, and the use of diverse domestic energy sources, including hydrogen, to reduce oil dependency.
Hydrogen has a high gravimetric energy density—fuel-cell vehicles on the road today can travel more than 300 miles with 11 lb of hydrogen and emit zero pollution from the tailpipe. However, current hydrogen-powered vehicles rely on high-pressure hydrogen storage tanks, which limit infrastructure practicality. Furthermore, the use of 700-bar (700 atmospheres of pressure) H2 gas is inefficient due to compression losses.
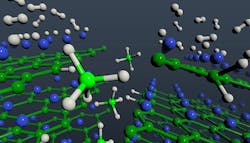
A hydrogenation mechanism that directly forms magnesium borohydride avoids issues known to inhibit the refueling speed for hydrogen vehicles. Hydrogen molecules (gray) dissociate on exposed magnesium (blue) layers of magnesium diboride and migrate to boron (green) edge sites to form borohydride units (BH4, center, light green and light gray).
Solid-state hydrogen storage in complex metal hydrides may offer much more compact onboard storage and reduced operating pressures. However, complex metal hydrides often have poor kinetics and multi-step hydrogenation pathways that are not well understood.
In the new study, the team found that in the initial stages of hydrogen exposure, MgB2 can hydrogenate to Mg(BH4)2 without forming intermediate compounds. The intermediates are known to inhibit the speed at which a hydrogen vehicle can be refueled, so avoiding them is an important development toward making MgB2 practically viable.
"We showed that if you can combine spectroscopy, first principles calculations, and kinetic modeling, it's possible to understand the reaction pathway and specific chemical mechanism in a way that hasn't been done before," says Tae Wook Heo, LLNL materials scientist. The research team also discovered that MgB2 hydrogenation occurs in two separate reaction stages as hydrogen molecules split and migrate to exposed edges in the material.