Solid-State Batteries for EVs: The Key to Long-Distance Driving?
Seems like everyone is getting into the race to build electric vehicles. This past February, James Dyson made the decision to build more than just high-precision vacuums and fans and instead focus on electric vehicles (EVs). Dyson plans to construct three EVs by 2020, the Financial Times reported, with an estimated $2.8 billion project. Much like Elon Musk, Dyson looks to shake up the EV market by using solid-state batteries instead of the lithium-ion versions.
According to the Financial Times, the trio of cars would be non-sports car high-end models which would scale in production aimed toward the mass market. The vehicles will be constructed out of light materials just like the BMW i3 EV carbon fiber body. The key is the solid-state battery which, theoretically speaking, will be able to hold more of a charge compared to lithium-ion batteries.
Dyson, famous for its vacuums and fans, aims to build electric vehicles by 2020 using solid-state batteries. (Image credit: Autocar UK)
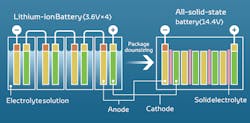
The main difference between the batteries is that lithium-ion batteries use liquid electrolytic solution to regulate flow, whereas solid-state batteries use a solid electrolyte. The electrolyte is the conductive chemical mixture that allows flow of the current between the anode and cathode. Using a solid electrolyte will provide a smaller size with higher energy density, longer lifespan, and increased safety. Solid-state batteries theoretically could store twice as much energy as a lithium-ion battery. For example, if the battery of Tesla Roadster were replaced with a solid-state battery, it could help double the 620-mile range from its 200-kilowatt-hour battery.
Dyson isn’t the only car manufacturer investigating this type of battery. However, production problems hinder solid-state battery adoption. Fisker EMotion, another high-end EV car which made its debut at CES 2018, is said to support the technology as well. Still, both Dyson and Fisker have stated that their first batch of cars will use lithium-ion before implementing solid-state batteries, due to problem of producing the latter on a large scale.
Solid-state batteries have the potential to hold a higher charge in a more compact shape, potentially doubling the driving range of electric cars today.
Currently, finding the right material for solid-state batteries is key. According to Axios, Samsung and Dyson have collectively invested $65 million in Massachusetts-based Ionic Materials. The firm claim to be making progress into the technology. Its new material, a liquid crystal polymer, may be able to solve many of the current issues of mass production.
Ionic Materials claims that lithium ions move as fast or even faster through their polymer than they would through a conventional liquid electrolyte system. This helps demonstrate the stability of the company’s polymer material. Second, the polymer works at five volts and can be made simply and cheaply. Third, while most materials in solid-state research operate at about 60°C (140°F), the firm’s material works at room temperature.
The Department of Energy's Pacific Northwest National Laboratory (PNNL) has also been experimenting with lithium-metal batteries. PNNL, in conjunction with the Battery500 Consortium led by PNNL—which aims to develop smaller, lighter, and less expensive batteries—is to demonstrate a small lithium-metal battery that can re-charge about seven times more than batteries with conventional electrolytes.
Researchers at PNNL developed a novel electrolyte for vehicle batteries that successfully creates a protective layer around electrodes—so they won’t corrode—achieving significantly increased charge/discharge cycles. (Image credit: PNNL)
PNNL has applied a solid protective layer around the electrodes that yields significantly increased charge and discharge cycles. Lithium-metal batteries that can replace a graphite electrode with a lithium electrode would increase the battery capacity, since a lithium-metal battery has double or triple the storage capacity. The extra power could potentially enable electric vehicles to drive more than two times longer between charges.
The new electrolyte battery is patent-pending and was tested in PNNL's Advanced Battery Facility on an experimental battery cell designed to replicate the charge of a watch battery. The tested showed the lithium-metal battery was able to retain 80% of its initial charge after 700 cycles of discharging and recharging. By comparison, a battery using a standard electrolyte would only be able to maintain its charge for about 100 cycles.
The next step for the PNNL is to test the lithium-metal battery on “pouch” batteries developed in the lab. The represent the size and power of a cell phone battery. Ultimately the goal is to nearly triple the specific energy found in batteries that power today’s electric cars.