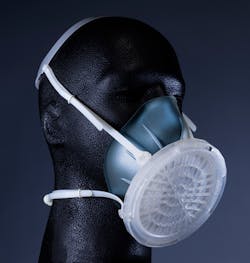
Thanks for Sharing: Reusable Open Source Hardware Respirators
Editors Note: Over the course of this week, Machine Design will be sharing a series of articles addressing the development and distribution of the COVID-19 vaccine—and the obstacles surmounted along the way. Be sure to check out the other installments below:
- Manufacturers Were on Point in the Race to a Vaccine
- Speed Bumps on the Road to Delivering COVID Vaccines
- Global Manufacturers Fine-Tune Strategies
- COVID Vaccine Moves to the Front of the Supply Chain
At a Glance:
- A group of engineers collaborate to design and produce a respirator to help offset a shortage of personal protective equipment during the pandemic.
- The particulate filtering facepiece respirator, also known as the OSR-M1, rivals the industry’s gold-standard N95 respirator.
- The designers use distributed production methods such as additive manufacturing to speed up time to market.
- Time constraints impede the OSR-M1 respirator’s probability of full certification and regulation.
When the World Health Organization declared COVID-19 a pandemic on March 11, reaction to the unbridled spread was raging: President Donald Trump hadn’t been tested yet, but had already invoked the Defense Protection Act to force General Motors to manufacture ventilators. Travelers would not be permitted into the United States from Europe for 30 days.
The state of New York declared a “containment zone” in New Rochelle, a city north of Manhattan. San Francisco called a moratorium on evictions. Tech giants in the Bay area urged employees to stay home. Tom Hanks and his wife, Rita Wilson, were in self-isolation. Coachella was rescheduled. The NBA suspended the season…
The world as Matt Carney knew it was in flux. Like many across the nation, he watched as the pandemic lay siege to the practicalities of everyday life and wanted to help. Globally, supply chains had stalled and production capacity for personal protective equipment (PPE), including medical-grade masks and respirators, was creating a health emergency that was revealing fundamental shortcomings in manufacturing while leaving America critically exposed. But it wasn’t until hospitals called on the public for 3D-printed face masks or respirators that Carney took action.
A mechanical engineer with a freshly minted PhD in biomechatronics from the MIT Media Lab, Carney was troubled by the idea that companies with FDM (fused deposition modeling) 3D printers would print face masks that would not necessarily meet the engineering properties or the U.S. Food and Drug Administration (FDA) clearance needed to be fit for purpose.
“I was really nervous about the facing of the devices because 3D printing, for the most part, is not a scalable process,” said Carney, adding that the effectiveness of a respirator is determined by two factors: the filtration efficiency and fit. “My biggest concern was companies were going to print these masks, give them to people who were going to put them on, and then they could fail at the worst possible time…I complained about it and then thought, I really can’t complain without offering solutions.”
An internet search led him to HelpfulEngineering.org, a volunteer-run crisis technology incubator, which Carney describes as “a gigantic Slack channel” and effectively a global hackathon corralling thousands of people’s skills in an effort to respond to the pandemic. Further raking through online threads connected him with Che-Wei Wang, a designer with expertise in computational and generative design and fabrication technologies, who started a private channel for an injection-molded facemask.
“This group of mostly engineers and designers was looking to leverage injection molding as a mass-manufacturing process for generating PPE,” recalled Carney. His own knowledge of 3D-printed materials stemmed from a stint as a product design engineer at IDEO, where the internals of a smartphone were built with injection-molded components: “The thinking was that if we could turn on idle manufacturing—everything had been shut down—then we could take advantage of the material properties of elastomerics to make a reusable, air-purifying elastomeric respirator that would hopefully use less filter material as well as use a broad range of filter material.”
Elastomeric respirators with facepieces made of synthetic or rubber material can be reused, cleaned, disinfected or stored. As a respiratory protective device alternative to the N95, the gold standard among filtering facepiece respirators, elastomeric respirators are equipped with replaceable filter cartridges. Some types offer higher assigned protection factors (APF) than N95 respirators while allowing for free breathing. An N95 with an APF of 10, for example, means the N95 reduces the aerosol concentration to 1/10 of that in the room, and equates to blocking 90% of airborne particles from being inhaled.
Depending on the manufacturer, filtering facepiece respirators such as the N95 have different filtration efficiencies for the most penetrating particle size (0.1 to 0.3 micron). Carney points out that air-purifying respirators, by comparison, test to a 0.075 micron—an important distinction relative to the COVID-19 particle, which is thought to be 0.1 micron in size. “Technically speaking, the coronavirus is smaller than the allowable particulate size of the N95 and surgical masks,” Carney pointed out.
The group had 3D printed and tested various respirator prototypes culled from open source design repositories and were in the process of reviewing their best options. Carney recalled the group’s frenetic back-and-forth texting at the time: “It was Saturday when I hopped on the group chat. Someone suggested we should get the prototype done by Monday. Another member said, ‘There’s no way we’re gonna get this done by then.’ I remember saying, ‘Nah man, we got this.’”
Open Source Isn’t Free-For-All
In an open sharing framework—using open source software and open source hardware—there’s a lot of free sharing of ideas, Carney noted. At first, the group had more than 30 people collaborating at any time, with smaller groups splitting off to work on parallel efforts and then recombining at stand-up meetings to compare progress on prototypes.
On the one hand, the initial forum provided a group of makers carte blanche to share knowledge, documentation and design files, and created an environment for rapid iteration and problem solving. On the other, the participants didn’t know the level of expertise anyone could contribute and, as a result, the informal group evaluated resources on the fly.
Meanwhile, progress stalled as the team sent out multiple respirator prototypes to labs for quotes. Even though tapping into connections at SolidWorks procured help with document control, cloud-based sharing and bottlenecks persisted. Team members were working off incompatible software and modeling computer-aided engineering design (CAD) programs: specifically, Fusion 360, SolidWorks, NX and Creo.
“Not a single one shared a native file,” Carney recalled. “I mean, here’s a global pandemic. We have a global team of engineers working tirelessly to solve this problem, yet we’re being held up by document control, versioning and branching of design solutions—all really critical to making it happen.”
A Florida-based medical lab, ATOR Labs, offered respiratory protective device testing to help out with the prototype’s breathability, including inhale-exhale resistance and residual CO2 tests in line with National Institute for Occupational Safety and Health (NIOSH) certification. Carney also harnessed his relationships at MIT. “Through MIT Media Lab we connected with AFFOA [Advanced Functional Fabrics Association of America], who offered filter testing resources,” he said.
Recognizing the dangers of feature creep and to focus the direction of the design, Carney stepped into the leadership role. Aaron Cantrell, a design engineer and principal at Cofab Design, a physical design consultancy in Holyoke, Mass., stepped up as co-lead. In defining its design goals, the formalized team would set out to build a filter media-agnostic, reusable elastomeric respirator which would become the Open Standard Respirator Model 1 (OSR-M1).
Open Source Ethos
The defining assumption of open source software (OSS) is that the original source code is released under a license in which the copyright holder grants users the right to use, change or distribute the software for any purpose. Today, Linux is among best-known open source examples; its interface underpins many popular systems, ranging from Android phones and Chromebooks to digital storages devices, cameras and wearables.
A 2017 Linux Kernel Development report claims open source operating systems dominate the supercomputer landscape, with 99% running on Linux, 90% of public cloud computing services, 82% of smartphones and 6% of embedded systems.
Taking cues from the OSS ecosystem, open source hardware (OSHW) fosters an open exchange of product design. Readily-available components and materials, standard processes and open infrastructure are at the core of OSHW projects, including the design, schematics and bills of material, as well as the software driving the hardware. In exchange for being accessible and free to share, the original sharer may gain feedback and potential improvements on the design.
OSHW gained ground in the mid-2000s when companies such as RepRap (3D printing), Arduino, Adafruit and OpenCores sprouted across the globe. Relative to OSS, there’s been about a 20-year lag in development, but the field has since exploded. “You can see that shift in Google Scholar citations, where the term ‘open source hardware’ is following the same exponential curve as open source software did,” noted Joshua Pearce, a professor of electrical and computer engineering and director of the Michigan Tech Open Sustainability Technology (MOST) Lab.
However, Pearce added that unlike OSS, which is the end in itself, OSHW costs money to prototype and manufacture. Beyond electronic hardware, OSHW is associated with tangible outputs, such as medical equipment and machine and vehicle components.
When stay-at-home orders and strict quarantine measures during the pandemic caused disruptions in global supply chains, it presented an opportunity for distributed manufacturing to fill unmet needs, Pearce observed. The author of Create, Share, and Save Money Using Open-Source Projects, he is an ardent advocate for accessible, collaborative technologies that respect user freedom, particularly as it applies to open source 3D printing and electronics in scientific equipment design.
Companies like Lulzbot and Creality have built their reputations on open source firmware, with hardware catching up, said Pearce, adding that the rise of 3D printing has catalyzed distributed manufacturing with the 3D printer itself being the best example.
Similarly, Harris Kenny, who runs market intelligence for OSHdata, a community that has certified more than 1,000 projects according to a common set of open source principles, drew on firsthand experience in describing the galvanizing effect the pandemic had on the OSHW ethos. As a volunteer on the Make4Covid project, Kenny helped raise $500,000 over the course of five weeks for PPE. The funds were primarily intended for 3D-printed face shields (“because they got the job done”) and fabric masks (“they had a reference design, and it could be made by anyone who had fabric in a needle or sewing machine”). From Kenny’s perspective, anyone with a 3D printer was able to participate, and Make4Covid showed that distributed manufacturing and distributed knowledge can make a difference.
Still, Kenny acknowledged there were challenges. Things were more complicated when it came to creating turnkey medical equipment, he said, in part because the designs’ firmware were “locked up.” Even if companies were to make designs available, makers would need access to injection molding facilities before requirements for product certifications and regulatory compliance kicks in. “You know, making sure the device isn’t going to kill somebody,” Kenny said.
Rallying the Network
Since the geometry of the filter component in the OSR-M1 is tied to that of the respirator, development was simultaneous. The synchronized work of testing and building enabled an agile development process. “As parts came back, we learned something from those experiments; we knew that some configurations were already changing and we were able to quickly grab the best parts of all the different designs and assemble them into a working system,” said Carney. “Early and consistent testing of critical features allowed us to have confidence in our design.”
The team also tapped into their networks. One team member, Philip Brown, an assistant professor at Wake Forest University and Medical Center in Winston Salem, N.C., was keen to improve PPE at the facility and solicited a financial contribution for soft tooling. Another, Jesse Jarrell, a product designer and founder of Kaos Softwear—a producer of injection-molded silicone body jewelry based in Portland, Ore.—became a major contributor, producing the low-volume injection-molded silicone facepieces. Meanwhile, Bjørn Sparrman, a researcher at MIT’s Self-Assembly Lab and spinout Rapid Liquid Printing, offered support printing silicone 3D-printed facepieces. Formlabs, a 3D printing manufacturer, donated resin. The Advanced Digital Design and Fabrication (ADDFab) lab at the University of Massachusetts Amherst pitched in to produce 3D-printed parts used for casting silicone prototypes.
“We were able to leverage advanced printing techniques to test out various parts of the design,” said Carney. “We tested face pieces with real sealing characteristics at ATOR Labs in Florida very early in the design process. We then went to 3D-printed molds—so we could cast silicone-like materials and achieve higher resolution in the 3D printing process.”
By the end of April, the design was frozen. “Things started to get serious, and we continued to push to identify filters,” he recalled. In doing so, the team was able to rapidly iterate, opting for a standardized interface between the base piece and the filter housing.
Open Standard Respirator
Understanding the safety requirements and relative effectiveness of personal respiratory protective equipment is key to the output success of the OSR-M1. The FDA regulates surgical masks and N95 respirators differently based on their intended use. Surgical masks are regulated devices that may come with face shields. They are disposable and can be effective in blocking splashes and large-particle droplets.
However, due to their loose fit, surgical masks don’t provide complete protection from germs and other contaminants. By comparison, the N95 respirator is also a single-use protective device, but its design achieves a very close facial fit and provides very efficient filtration of airborne particles.
N95s are tested against NIOSH Standard 42CFR 84, a Federal regulation for certifying air-purifying particulate respirators. A respirator patterned after an N95 would need to be designed to achieve very efficient filtration of airborne particles, as well as have a tight fit to ensure air flows through the mask and not the sides. Fortunately for Carney’s team, timeous legislation and research allowed them to forge ahead with designing a device that would meet a comparable level of protection under pandemic constraints, and without compromising requisite criteria.
As of April 10, 2020, due to a dire shortage of N95 respirators, the FDA issued an emergency use authorization (EUA) to decontaminate compatible N95 or N95-equivalent respirators for reuse by healthcare workers in hospital settings. Other research reported in the Journal of the American Medical Association (JAMA), found that there was no significant difference in the incidence of laboratory-confirmed influenza among healthcare personnel with the use of N95 respirators (8.2%) versus medical masks (7.2%).
The OSR-M1 is not the coolest looking device and will likely undergo design improvements in future iterations. (“Yeah, it’s not a lifestyle device—it’s a functional device,” conceded Carney.) But in its current form, the OSR-M1 can be characterized as somewhere between a disposable respirator and a traditional industrial elastomeric respirator that’s lightweight and form-fitting with a good seal. Its modular design is a distinguishing feature that accommodates face pieces in varied sizes. “From a supply chain point of view, we can swap out manufacturers as we need,” Carney said. “We kept to this concept of ‘filter media agnostic’ so we can have multiple filter suppliers and we can swap out [filters] based on changes in the supply chain.”
Who Holds the IP?
Carney, who happens to build robotic prosthetic legs for a living, was well aware that hitting snags would be par for the course in developing a respirator rivaling the industry workhorse. There was an additional reality check, however: Producing the respirator in an open source channel is not a bet against pandemic time constraints, but a bet against time to market. In raising funds for production, Carney’s team applied for multiple grants and created a non-profit, Open Standard Respirator. Rather than knocking on doors for donations, the OSR team found it easier to raise money for investment and opted to also create Open Standard Industries, a C-corporation, as it provided a mechanism to use debt to scale production or to sell equity.
“Going this route was a surprise to us because we weren’t trying to start a company,” reflected Carney. “We just wanted to come up with a design that a manufacturer would take and run with, or make it for people.” But while shopping around for a manufacturer that would want to produce it, he found instead that manufacturers wanted to “take the design and fully lock it up by buying all the intellectual property (IP), or hold onto it and not release it at all.” One company liked the respirator idea so much that it responded that it might work on its own version of the respirator. “Ultimately we found that we just need to make it, and drive it,” said Carney.
If the OSR-M1 proves to be as robust as Carney believes it to be, then a hybrid business model approach could prove to be effective. Pearce maintains the time for “locking down IP for 20 years” is a thing of the past. “The number of people who see the potential for distributed manufacturing have increased massively during the pandemic,” he said. “When we go back to normal times, they didn’t lose the skills they acquired, they didn’t lose that information and all the designs that were created when we have millions of engineers locked in their apartments isn’t going away. So, you can try to patent that if you like, but you’re up against a bunch of people that can make their own things.”
For the most part, people don’t make things for hospitals; they make things for themselves. “It’s going to be a challenge to the business community,” Pearce surmised. “If manufacturers make injection-molded plastic dinosaurs, and hobbyists or engineers can make it at higher resolution from their basement and make it more functional, what would be the need to buy commercially produced products? Maybe the industry will need to think about the next step up, instead of trying to trademark the Triceratops.”
As Carney and his partners navigate the business end of producing the OSR-M1, they gain a fuller appreciation for the resources needed for high-volume manufacturing. “You really need to put pretty serious money in; it’s hundreds of thousands of dollars to build up the tooling for injection molding,” he noted. “And the field got so crazy with sourcing non-woven polymer material, the fundamental component of the filter. There’s a lot of volatility and a lot of insecurity in the supply chain and so you really have to purchase large orders that actually get enough attention to drive things forward.”
Meanwhile, the respirator team has shortlisted two filter manufacturers that both supply filters with efficient filtration (“hitting N95 or better”) and meet the filter criteria for breathability, residual CO2 and inhale-exhale resistance. The OSR-M1 can technically be designated an air-purifying respirator. According to Carney, it has garnered attention from filter manufacturers because it uses about four times less filter material than a typical disposable respirator and, over time, is cheaper to use than comparable respirators. “If we can get it into production, we instantly expand the filter supply capability of these manufacturers,” he estimated.
Certification in Progress
Passing mechanical testing is one half of the development equation. The other is certification. The respirator team produced low-volumes of the OSR-M1 for testing and enlisted several hospitals, including Wake Forest Baptist Health in Winston-Salem, N.C., and Boston Children’s Hospital. Based on fit, feedback was positive, yet orders haven’t been rolling in.
Carney noted that healthcare facilities were dubious about the fact that the respirator was open source and not certified. They also expressed concerns that the market has become flooded with low-quality devices. “It’s confusing from a supply chain point of view because there are so many knockoffs,” he conceded.
The feedback no doubt played into the team’s mentality since they changed their original thinking that “people would just make it out of the kindness of their heart,” said Carney. “We realized we couldn’t do it that way because hospitals need NIOSH-certified devices, and NIOSH certification means there needs to be a manufacturer on record for that manufacturing process, and that manufacturer has to retain the design history files and the full quality management system.”
Medical approval, particularly in the U.S., is a challenge for new devices, noted Pearce, as FDA approval of the design and function of a medical-grade device is coupled to the manufacturing site or location. The workaround is to pursue an ISO 13485-compliant quality system within the manufacturing process. Working with a local manufacturing supply chain that’s compliant with a quality management system for the design and manufacture of medical devices is required for NIOSH certification, Carney said.
Even if the process has compelled the OSR-M1 maker team to change course in unexpected ways, the original goal to get respirators to healthcare providers and essential workers remains in sharp focus. “We just had to pivot a little bit based on the realities of where we can raise money,” Carney said. “We do think that there needs to be reference designs out there for people to understand how to make these things better, and we also want to be sure that we can provide a quality solution to people who need it.”
Editor's Note: Matt Carney can be contacted via the Open Standard Industries website.
About the Author

Rehana Begg
Editor-in-Chief, Machine Design
As Machine Design’s content lead, Rehana Begg is tasked with elevating the voice of the design and multi-disciplinary engineer in the face of digital transformation and engineering innovation. Begg has more than 24 years of editorial experience and has spent the past decade in the trenches of industrial manufacturing, focusing on new technologies, manufacturing innovation and business. Her B2B career has taken her from corporate boardrooms to plant floors and underground mining stopes, covering everything from automation & IIoT, robotics, mechanical design and additive manufacturing to plant operations, maintenance, reliability and continuous improvement. Begg holds an MBA, a Master of Journalism degree, and a BA (Hons.) in Political Science. She is committed to lifelong learning and feeds her passion for innovation in publishing, transparent science and clear communication by attending relevant conferences and seminars/workshops.
Follow Rehana Begg via the following social media handles:
X: @rehanabegg
LinkedIn: @rehanabegg and @MachineDesign